RECOVERY trial: the race to find treatments for COVID-19 patients
The world’s largest randomised clinical trial of potential coronavirus treatments delivered the first major breakthrough in the COVID-19 response
At the start of the COVID-19 pandemic, nobody knew which, if any, treatments would be effective. The RECOVERY trial is a large, randomised controlled trial of possible treatments for patients admitted to hospital with COVID-19. Patients who agree to be in the trial are randomly assigned to groups which either receive a specific treatment(s) in addition to the usual care delivered by their hospital, or receive the usual care alone. Researchers can then compare what happens to those who receive the treatment with what happens to those who don’t.
The trial is led by Professor Peter Horby in the Nuffield Department of Medicine which has world-leading expertise in infectious diseases, and by Professor Martin Landray in the Nuffield Department of Population Health which has pioneered novel approaches to delivering effective and streamlined randomised clinical trials.
Around 29,000 patients in 176 hospital sites across the UK have been randomised to nine treatment arms, or to receive no additional treatment. The Oxford University coordinating team comprises dedicated doctors, data analysts, medical statisticians, trial managers, and communications specialists who have pooled their expertise to deliver the trial at unprecedented speed; it took just nine days from conception to launch. During the first wave, over 10,000 patients were recruited in just two months, making it the fastest-recruiting randomised controlled trial and, with the second wave upon us, over 6,000 patients have been recruited in the past three weeks.
The trial is deliberately inclusive: the youngest participant was less than six months old and the oldest over 100 years; 1/3 are women; and 1/6 are of Black, Asian or Minority Ethnic (BAME) background. The RECOVERY trial is unusual in its commitment to include pregnant women to ensure that they have equitable access to trial treatments.
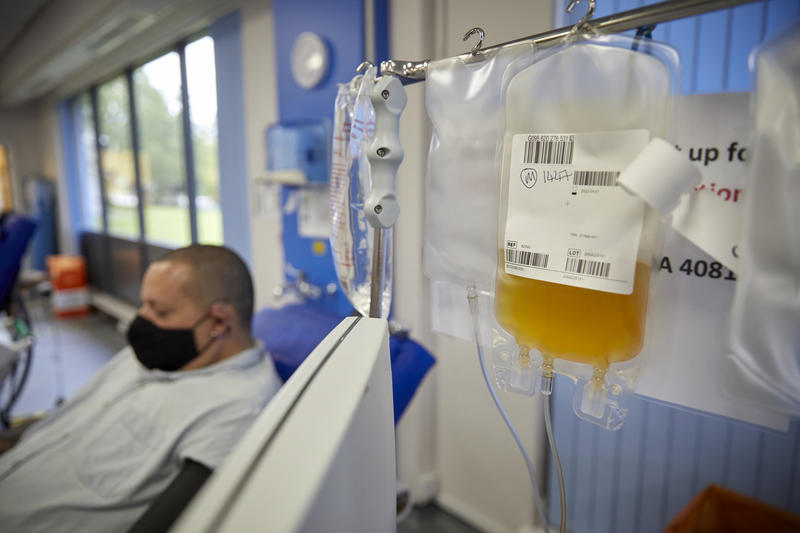
Convalescent plasma donation in August 2020
Courtesy of NHS Blood and Transplant
The results so far
In 100 days, the RECOVERY trial provided three sets of results enabling change in global practice.

The first major breakthrough in the COVID-19 response was announced in June – the finding that dexamethasone (a steroid treatment) saves the lives of severely ill COVID-19 patients. The drug reduced the number of deaths in patients receiving breathing support with a ventilator by 1/3, and in patients receiving oxygen without a ventilator by 1/5. Within hours of these results being announced, doctors started using dexamethasone to treat patients in the UK. It is now widely used across the world and is estimated to have saved around 650,000 lives globally.
Learning that a treatment is not effective is important as it protects patients from potential harm and avoids wasting resources. Hydroxychloroquine (a treatment for malaria) had been widely used to treat COVID-19 patients and championed by high-profile figures such as Donald Trump, despite a lack of evidence of its efficacy. In early June, the RECOVERY trial announced the finding that hydroxychloroquine did not reduce the number of deaths or the length of time patients with COVID-19 spent in hospital, nor did it benefit patients in any other way.
In late June, the RECOVERY trial also found that there is no beneficial (or harmful) effect of lopinavir-ritonavir (an antiviral treatment commonly used to treat HIV) in patients hospitalised with COVID-19. This treatment had previously been recommended in many countries.
RECOVERY provided sufficient evidence for clinical guidelines to be updated rapidly, reversing clinical practice from widespread use of hydroxychloroquine and lopinavir-ritonavir and limited use of dexamethasone to the opposite pattern.
The trial has recently demonstrated that the antibiotic azithromycin is also not an effective treatment for patients hospitalised with COVID-19, though it had been widely used to treat COVID patients because of its theoretical potential to reduce lung inflammation.
Convalescent plasma (collected from donors who have recovered from COVID-19) has been given to hundreds of thousands of patients admitted to hospital with COVID-19 in the US and elsewhere without reliable evidence of its efficacy. On Friday, the RECOVERY team announced that convalescent plasma does not improve the chances of survival of patients in hospital. The RECOVERY trial of convalescent plasma was the largest trial of this potential treatment, and has now provided definitive evidence that will enable clinicians and trialists to focus resources on treatments that may be beneficial.
Ongoing research
The trial is continuing to test other treatments – aspirin, colchicine (a commonly used anti-inflammatory), tocilizumab (an anti-inflammatory treatment given by injection), and Regeneron’s antibody cocktail (a combination of monoclonal antibodies directed against coronavirus).
Given the success of the Phase III RECOVERY trial, the UK government has increased its investment so that new treatments can be tested through RECOVERY+, including treatments tested in Phase II (smaller) and Phase III studies. The team are now preparing to deliver RECOVERY internationally, with new sites being established in Indonesia, Nepal and Vietnam.
The trial is the best-performing trial anywhere for COVID-19 and is being promoted as the way forward for drug trials. Academics from across the world are drawing lessons from the pioneering design of the trial which minimises the impact on frontline teams by integrating the research with clinical care and making best use of national data sources. The trial is also generating wider understanding of the importance of clinical trials in providing reliable evidence, and has been described as a ‘beacon of excellence’.

Professor Peter Horby, Professor of Emerging Infectious Diseases and Global Health
The RECOVERY trial set out to find effective treatments for COVID-19 that were widely available, inexpensive and could be easily used worldwide. Dexamethasone, the first drug to be shown to improve survival in COVID-19, perfectly met this need. This was an extremely welcome result. Dexamethasone is now used worldwide and is estimated to have already saved around 650,000 lives globally. We are eagerly anticipating results for other trial treatments and hope to find another beneficial therapy.
The RECOVERY trial is an extraordinary collaborative effort involving many thousands of doctors, nurses, pharmacists and research administrators at hospital sites across the UK, as well as the dedicated trial team based at Oxford University. At this very challenging time, we are incredibly grateful for the hard work of NHS staff and the huge contribution made by patients across the whole country. Thanks to their efforts, we have now delivered five significant results and will soon find out whether other treatments are beneficial for patients in hospital.

Professor Martin Landray, Professor of Medicine and Epidemiology


